Stories From The Field : Capacity Building in Action
Partnering for Impact: Capacity Building and Digital Solutions Aligned with Global Health Guidelines

The Importance of Continuous Training in Women’s Cancer Care
Rwanda is on track to eliminate cervical cancer by 2027, three years ahead of the WHO’s global goal. This achievement is rooted in strong education, continuous training, and effective partnerships and usage of digital tools. As Rwanda nears this milestone, the focus is shifting to sustainability: ensuring that the systems and skills built during the elimination process endure well beyond 2027. A key step toward this future is the integration of cervical cancer screening and management into existing healthcare services, such as antenatal care, family planning, and HIV clinics. For example, Rwanda is moving from on-the-job training to embedding screening and management protocols into pre-service (in-school) curricula for health professionals. This ensures that new generations of providers are equipped from the start, and that ongoing training remains a national priority.
Sustaining high-quality care, maintaining awareness, and leaving no woman unscreened will be essential. Rwanda’s experience with mass cervical cancer screening and the learning gained can inspire and support other African countries planning similar efforts.
Raising the Bar: How Regular Training and Mentorship Are Accelerating the End of Cervical Cancer
Dr Emmanuel Manirakiza: Gynecologist, Obstetrician, and Lead of the Elekta Foundation Rwanda Mission 2027 Team always highlight, “Ongoing capacity building ensures that frontline providers maintain high standards of diagnosis and treatment. Regular mentorship and refresher courses enhance clinical confidence, improve the quality of care, and ensure adherence to national and WHO guidelines. This continuous professional development strengthens service consistency, reduces diagnostic errors, transmits new innovations, and contributes to higher treatment success rates, thereby accelerating progress toward the full elimination of cervical cancer.
The Presence of HPV virus and Precancerous lesions
Through our extensive fieldwork and collaboration with Dr. Emmanuel, we have gained a clear picture of the viral landscape in Rwanda. Our screening programs typically reveal an HPV prevalence rate of approximately 20% among the women we test.
We observe that this rate is not static; it fluctuates between geographical districts, often influenced by social factors such as sexual activity and the number of partners.
However, our field teams emphasize a crucial distinction to patients: testing positive for HPV does not mean a woman has cancer. While many virus strains exist, our work focuses on identifying the specific high-risk types—HPV 16, 18, and 45—that are the primary drivers of disease progression.
The data from our program districts validates exactly why mass screening is effective. While the virus is relatively common (20%), the progression to disease is much rarer when caught early. In the areas where we operate, we have identified a pre-cancerous lesion rate of 2.2%, compared to an advanced stage cancer rate of just 0.03%. These figures are a testament to the power of our “screen-and-treat” model. By identifying that 2.2% of women early, we can intervene immediately, preventing them from ever becoming part of the 0.03% statistic.
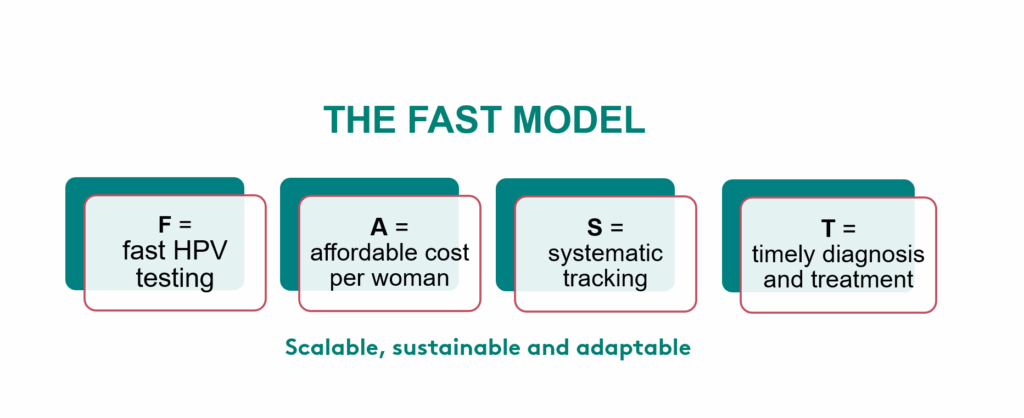
Reimaging HPV Screening for Every Setting
Population-based HPV screening in low-resource settings demands specialized, reliable HPV tests. At Elekta Foundation, we recognized this from the start and evaluated innovative self-sampling HPV solutions early on. Since then, we have been proud to work with our partner Gynius to introduce an HPV test that meets these needs and is now a key component of the Elekta Foundation co-designed FAST model, ready for use in low-resource settings worldwide.
The HPV kits we use are designed for flexibility and reliability. They support both point-of-care testing and high-throughput screening, making them suitable for mobile clinics as well as large-scale programs. Each kit includes built-in quality control to ensure samples are validated before processing, reducing errors and improving accuracy. The process uses PCR (Polymerase Chain Reaction), a proven technique that amplifies tiny amounts of DNA into millions of copies, enabling detection even from minimal samples. Combined with automated liquid handling platforms, these kits minimize human error, speed up workflows, and deliver timely results. By integrating these advanced HPV kits into the FAST model, we make cervical cancer screening scalable, secure, and sustainable, bringing life-saving care to communities everywhere.

Shared Commitment
Elekta Foundation and Gynius share the mission of making women‑centered cancer care more accessible. Long-term partnerships make this possible, they simplify collaboration and are key to achieving results and advancing patient-oriented health development.
“Through our strategic partnership, we are introducing products tailored for low‑resource settings that enable population‑based screening. Driven by continuous innovation, we focus on delivering solutions that integrate advanced software capabilities and telemedicine to support clinicians and patients wherever they are. With a partner like Elekta Foundation, whose deep expertise spans cancer care and women’s health development, we are ready to elevate women’s cancer care to new levels. Achievements in Rwanda already demonstrate this progress, we have introduced higher degrees of automation, strengthened post‑analytical data management, implemented room‑temperature logistics, and increased sustainability through fewer consumables, saving pipette tips, and recycling COVID‑era PCR instruments,”says Dr. Huaqing Li, CEO, Gynius.
From Theory to Action: The Master Trainer Behind Mission 2027
Euloge Nzobonankira is a driving force behind sustainable capacity building on the ground in Rwanda. Through a structured blend of intensive theoretical learning and supervised practical field sessions, he ensures local nurses and midwives are equipped with the precise expertise needed to identify and treat pre-cancerous lesions effectively.
This comprehensive training does more than build technical skills; it reshapes the narrative around cervical cancer. Euloge’s mentorship gives health workers the belief that the disease is no longer an insurmountable fate, but a manageable condition. This confidence is the key to scaling up access and achieving Rwanda’s ambitious goal of elimination.

